Policy Changes and Pharmaceutical Innovation Combine to Increase Naloxone Access
Naloxone, which reverses the effects of an opioid overdose, is a critical tool for responding to the opioid crisis. However, prior to the 2010s, two barriers hindered its widespread distribution and use in the United States. One was legal access: Naloxone required a prescription from a healthcare provider. Another was that naloxone was administered by injection and therefore required training for proper use.
In 2010, Illinois became the first state to adopt a dispensing naloxone access law (NAL) that permitted individuals to obtain naloxone directly from pharmacists, eliminating the need for an individual prescription. By 2015, another 35 states had implemented dispensing NALs. These policy initiatives were complemented by the introduction of Narcan, the first FDA-approved naloxone nasal spray, in 2016. This new formulation allowed untrained bystanders to administer naloxone.
In Using Policy and Innovation to Improve Life-Saving Access to Naloxone (NBER Working Paper 33105), researchers Evan D. Peet, David Powell, and Rosalie Liccardo Pacula examine the impact of dispensing NALs and Narcan and highlight an important interaction between the two. They demonstrate that Narcan’s introduction had a larger effect in states with NALs already in place, and the adoption of new NALs had a greater impact after the introduction of Narcan.
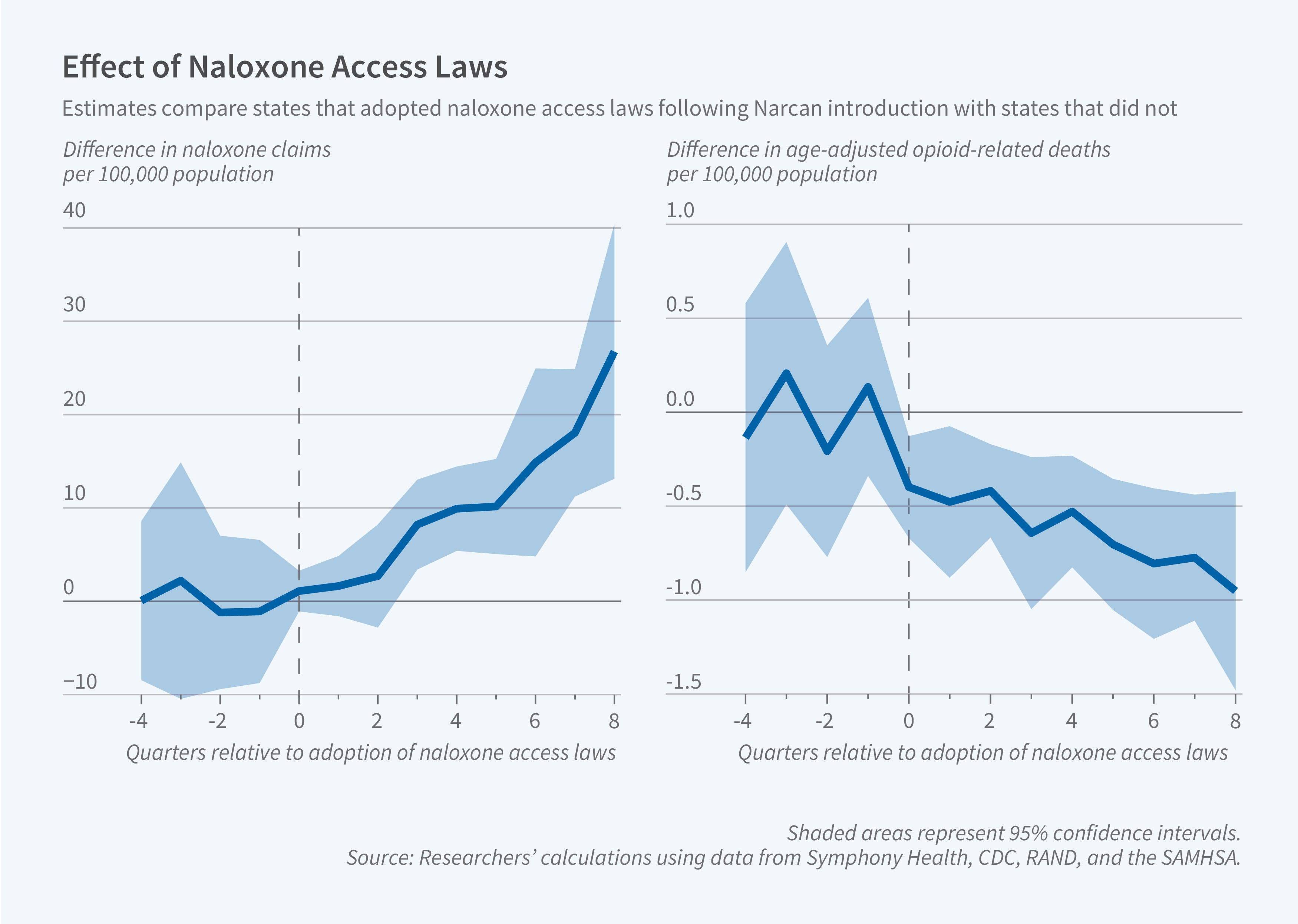
Naloxone access laws reduced opioid-related mortality after Narcan, a nasal spray, was introduced, but not before.
The researchers use pharmacy prescription claims data from Symphony Health to measure naloxone dispensing at retail pharmacies. They use CDC National Vital Statistics System mortality data to describe age-adjusted opioid-related mortality rates, focusing on mortality involving heroin, natural opioids, or semisynthetic opioids such as OxyContin.
Prior to the introduction of Narcan, the adoption of a dispensing NAL led to 1.4 additional naloxone claims per 100,000 residents per quarter, a 164 percent increase, but did not alter the rate of mortality from non-synthetic opioids. After Narcan was available, however, these states experienced dramatic increases in naloxone dispensing and reductions in opioid-related deaths. Sales of naloxone increased by 9.2 per 100,000 residents per quarter, while deaths fell by 0.143 per 100,000 residents per quarter (a 10 percent decline). Dispensing NALs that were adopted after Narcan’s introduction had pronounced effects: Claims for naloxone increased by 12.7 per 100,000 population per quarter and mortality from non-synthetic opioids fell by 0.635 per 100,000 per quarter (39 percent).
The researchers separately examine mortality from synthetic opioids such as fentanyl. A single dose of naloxone is typically insufficient to reverse the effects of an overdose from them. Neither NALs nor Narcan’s introduction reduced deaths associated with these more potent opioids.
Naloxone claims increased the most among consumers facing the lowest out-of-pocket costs, including Medicare and Medicaid beneficiaries. The researchers conclude that, while the combination of dispensing NALs and Narcan relaxed two key barriers to the use of naloxone, out-of-pocket costs remain an obstacle.
— Robin McKnight
The researchers acknowledge financial support from the Centers for Disease Control and Prevention (R01CE02999) and the National Institute on Drug Abuse (2P50DA046351).


